
Neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis are diseases characterized by the slow and progressive loss of one or more functions of the system nervous. These are seriously debilitating diseases which have heretofore been treated with poor results by the administration of purely symptomatic drugs. It’s one of those things that when it happens to you, it’s a final judgment. At least until today: there is a little flame of hope in Japan these days …
The number of people with neurodegeneration is dramatically high. Alzheimer’s disease affects around 50 million people worldwide[1], and in the absence of truly effective treatments, this number will increase dramatically due to the increase in average age and therefore the increase in the proportion of the population at risk. The World Health Organization estimates that 10 million people have Parkinson’s disease[2] and, like Alzheimer’s disease, the incidence increases dramatically after 65 years. Multiple sclerosis affects more than 2.8 million people[3], while ALS, with a sharp annual increase, paralyzes at least between 200,000 and 300,000 patients[4].
The effects are devastating: diseases like Alzheimer’s disease are progressive, with an average duration of 10 years, during which the autonomy of the patient continues to decline, requiring a kinship commitment and increasing costs. In addition, these patients are hardly ever hospitalized: more than 75% of care and support is provided by families facing a daily tragedy that has hitherto been insoluble. But science is fighting with all its might to change this situation, and it is finally succeeding, especially in the field of motor neurons. Especially since we have started to work on the flexibility of a drug that we have had for nearly half a century, ibudilast, which turns out to be full of good surprises.
The nervous system and its neurons
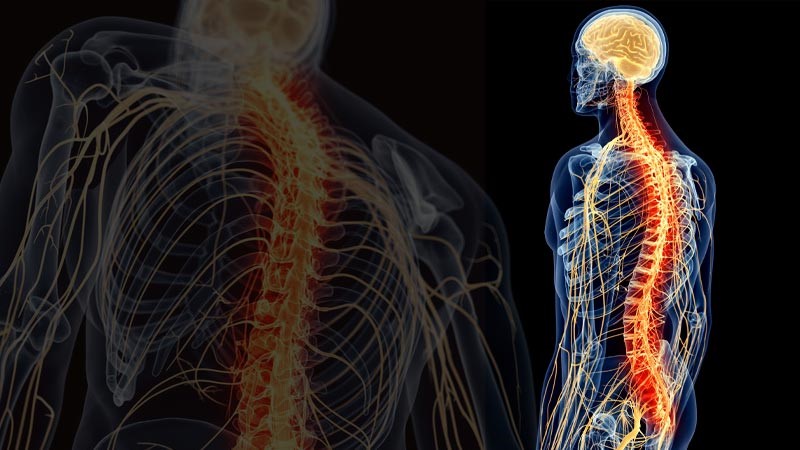
The human body is crossed by a complex structure, the nervous system, which exchanges the information necessary to activate the various organs of our body. Many of them work autonomously thanks to the nervous system. The system is divided into two subsystems: the central nervous system (CNS)[5], which affects the brain, cerebellum, spinal cord and brainstem, and the peripheral nervous system (PNS)[6], which controls the rest and is divided in somatic systems … The latter, voluntary or somatic, regulates the activities that we carry out consciously, such as for example using a pencil, kicking a ball, talking, etc. The first, autonomous, also called involuntary or vegetative, regulates automatic processes such as heart rate, respiration, metabolic processes, etc.
The first subsystem (CNS) is made up of neurons and nerve fibers in the brain, protected by the skull, and in the spinal cord, which is contained in the spine. The central nervous system is the laboratory that collects and processes signals from the peripheral system and whose commands to the peripheral system itself differ[7]. The peripheral nervous system (PNS), on the other hand, is made up of receptors and nerves that send information from the periphery to the spinal cord and brain. Unlike the central nervous system, which is protected by the skull and the spinal canal, the SNP has no protection[8].
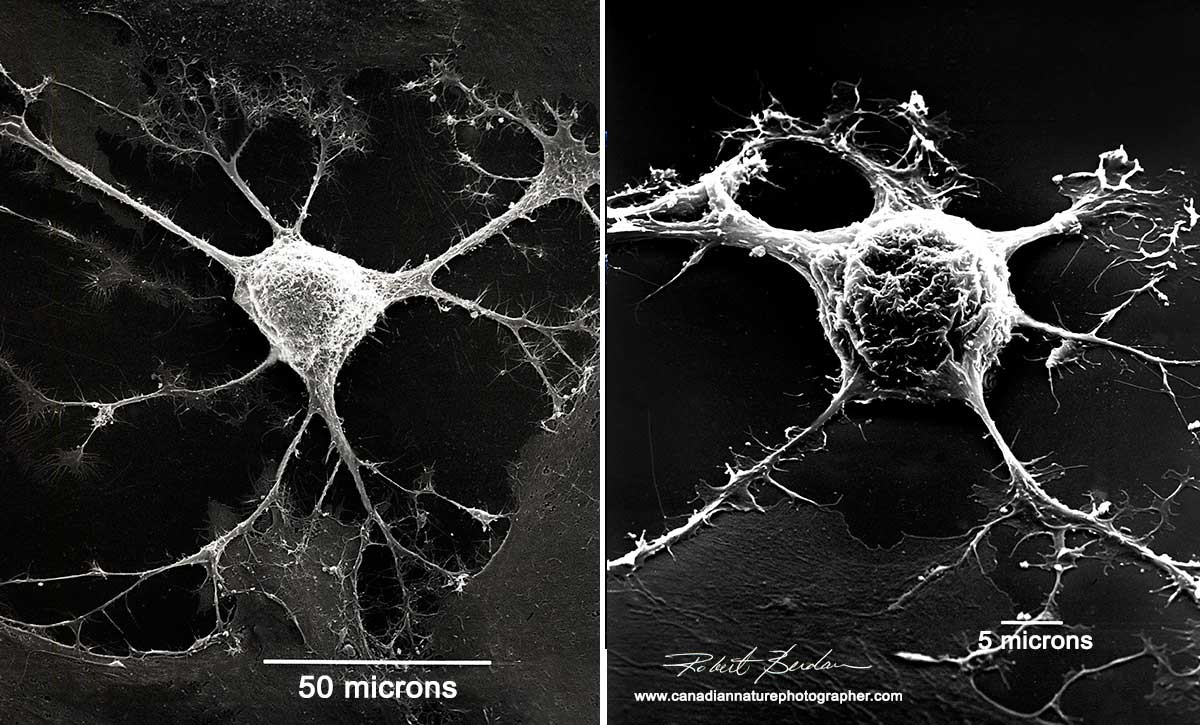
A nerve cell under an electron microscope[9]
Our body needs a dense communication network to allow the transmission of specific information for each automatic or conscious task. This network (the nervous system) is made up of tissues (nerve cells) that are associated, connected, transmitted and received through particles called neurons. They are highly specialized cells that can exchange messages thanks to two of their own characteristics, excitability and conductivity[10].
Excitability means that the nerve cell reacts to external stimuli (physical and chemical), which are converted into a nerve impulse. For example, a neuron in the inner ear is stimulated by a sound wave, that of the skin by heat or cold, that of the eye by light, the olfactory by a smell, or that of the muscle by the perception of effort or resistance[11]. Conductivity means that the nerve impulse generated by a nerve cell due to its excitability can be transmitted to other cells as an electrical impulse at cell junctions, called synapses[12].
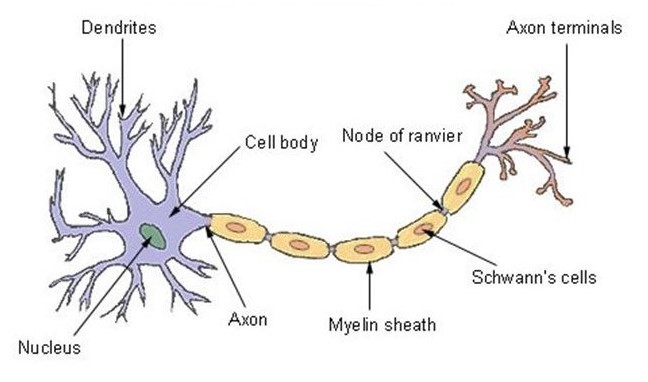
Morphology of a neuron
The neuron has one of the best-known structures in nature. For this reason, its design was copied by writer Arthur C. Clarke and director Stanley Kubrick to create the Discovery One spacecraft for the movie “2001 A Space Odyssey”[13]. The nucleus at the front is called the soma and contains the enzymes that make the neuron active[14]. The central parts, called dendrites and tubulars, are the branches that – like antennae – receive and decode signals from the nervous system[15].
The third region, the axon, is a sculpted appendix that can be up to three feet long – as in the case of neurons that control self-controlling muscles – or just a few microns long[16]. The axon is representative in transmitting signals from the center to the periphery and often has collateral branches which allow the axon to distribute information to different destinations at the same time[17].

The Discovery One spaceship from the movie “2001 A Space Odyssey”[18]
The axon therefore uses these branches which connect in synapses to allow the transmission of neuronal signals. These synapses are connections between the neuron and other cells responsible for transmitting the nerve message (impulse). The message is contained in a chemical released by axons and stored in vesicles before being sent[19].
The nerve fiber is therefore made up of the axon – the basic structure that allows the impulse to be conducted – and a sheath that covers it and helps to insulate and protect the nerve fibers and to increase the speed of transmission of the impulses. This shell is called myelin and is not found everywhere: neurons in the brain and spinal cord do not have this protection[20]. Myelin is an insulating substance with a lamellar structure made up of fat and protein molecules that externally covers the axons of neurons[21], the structure of which inspired the invention of the electric cell nearly 200 years ago[22].
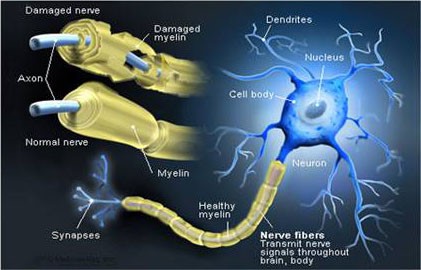
The functions of myelin are different: a) it allows the good transmission of nerve impulses and increases (if necessary) their speed; b) protects and nourishes the axon which envelops it[23]. Defective myelination of a nerve is the main cause of various neurological diseases: Sometimes the nerves are surrounded by damage or even loss of the myelin sheath – either due to infection or Metabolic or genetic reasons: whether it is because the loss of myelin causes nervous dysfunction and slows down or even blocks the transmission of messages between the affected neurons and therefore the brain commands to different parts of the body[24].
One of the most serious and well-known diseases caused by demyelination is multiple sclerosis, in which demyelinating lesions of the central nervous system appear in the brain and cause various symptoms such as pain, cognitive and motor dysfunction and ultimately , visual impairment[25].
Motor neurons and amyotrophic lateral sclerosis
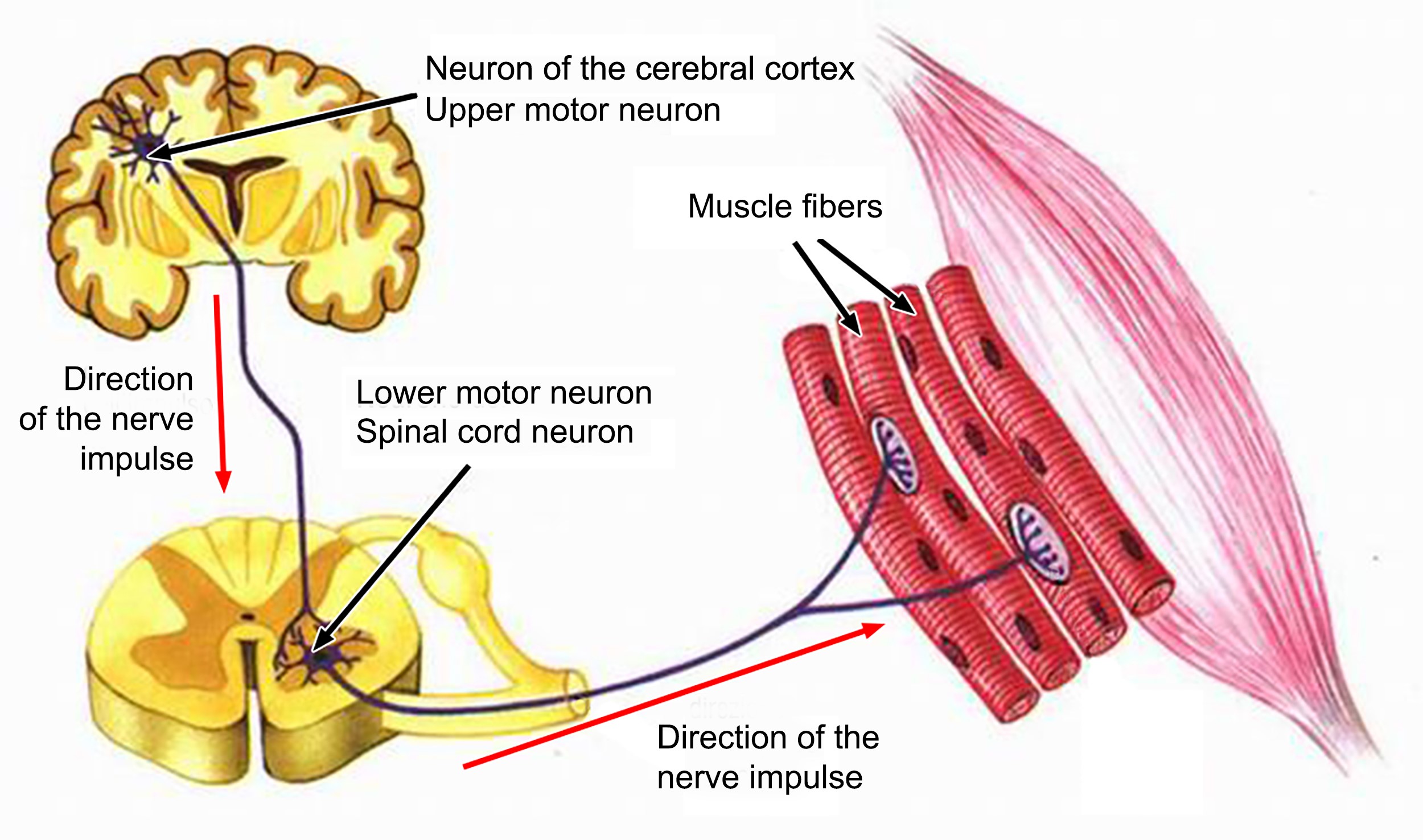
Like a spaceship, the body is an extremely complex machine powered by a certain type called motor neurons because they control the millions of movements with absolute precision that allow us to control our actions even in the most complex actions whose coordination is required by many functions. For this, there are sensory or afferent neurons which carry information from the sensory organs to the central nervous system; and intercalary neurons, which receive data from sensory neurons and transmit it to motor neurons. These latter, motor neurons (or motor neurons), specialize in transmitting motor and coordinated impulses to each individual organ on the periphery of the body[26] – an absolutely miraculous task.
The motor neuron is one of the largest cells in the nervous system, and there are two types: The first motor neuron – also called central – is the cell in the frontal motor cortex that sends a very long upward stretch. from the spinal cord, to reach the second motor neuron. The second motor neuron – also called peripheral – is the cell that emerges from the spinal cord, reaches the periphery, and innervates skeletal muscle[27]. A structure used by Carlo Rambaldi in the late 1970s to create the monster of the “Alien” film trilogy[28].
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, named after the first baseball champion diagnosed[29], is a progressive neurodegenerative disease in adulthood and belongs to a group of disorders that affect progressive degeneration of some type of motor neuron is common[30]. In case the degeneration affects the first motor neuron we have primary lateral sclerosis[31], in case the infection affects the peripheral motor neuron we have progressive muscle atrophy[32] – which, to be clear, affects the famous astrophysicist Stephen Hawking[33].
Instead, amyotrophic lateral sclerosis (ALS) is a condition in which the two types of motor neurons, the first and the second, gradually degenerate simultaneously until the patient dies[34]. The nerve fibers stop sending messages to the muscles, and over time these weaken, forming the first contractions, called fasciculations[35], then wasted or atrophied[36]. Eventually, the neural system completely loses the ability to initiate or control self-controlled movements.
The causes that produce ALS are unfortunately largely unknown, but what is certain is that they are determined by a number of causes. The belief that there is a genetic mutation at the base is gaining ground, but another decisive factor seems to be environmental or toxic-environmental: many studies suggest that pollution caused by certain metals or pesticides is the evolution of the disease[37].
Scientists are working on phenomenology – like the buildup of abnormal proteins in the cell or the inability of the cell to shed those diseased proteins independently[38]. Or the inflammatory state of glial cells that help nourish neurons[39]. Sometimes there is a defect in the intraneuronal signal transport mechanism[40] or there is an excess of glutamate[41].
We can discover growth factor deficiencies in nerves[42] or synapses or even in mitochondria, the DNA cells that carry the genetic message that determines whether we are animals or plants, reptiles or mammals, humans or women[43]. All of this drives scientists to despair: we are still trying to figure out what is going on and how, but we still don’t know why, but something has to be done to cure or stop the disease and therefore we are looking for a substance that will slow down the degeneration.
The phases of experimentation
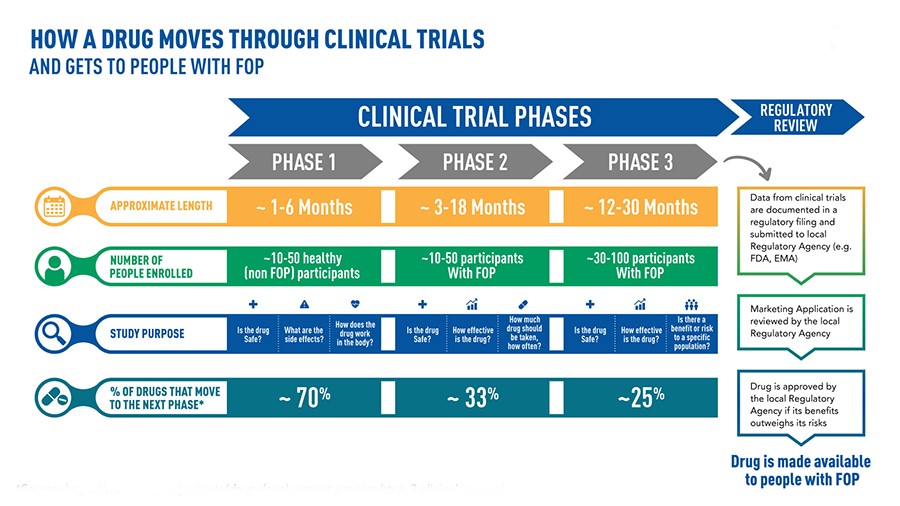
It can take over 10 years of careful planning and research, and even over $ 1 billion, to bring a drug from discovery of the molecule to commercialization[44]
When a pharmaceutical company believes it has discovered a particular drug, an experimental process that is rightly long and necessary to determine the safety and effectiveness of the new treatment begins[45]. In the laboratory, a so-called “preclinical” phase develops in which the degree of toxicity of the molecule is observed and the most efficient route of administration, absorption and subsequent elimination by the human body is evaluated. These are test-tube studies in which the molecule can interact with cell cultures or microorganisms. If the results show effective efficacy, we proceed to experiments on animal guinea pigs. When this phase confirms efficacy and shows tolerability, the actual experimentation begins[46].
Phase 1 – The administration will begin in a limited number of healthy volunteers, the main objective of which is to review the possible occurrence of side effects. We observe how the drug works in the human body and how the body manages it[47]. In cases where severe reactions are observed or the effectiveness is related to the use of excessive doses, the new medicine will be stopped. The race ends there.
Phase 2 – This phase examines the real therapeutic potential of the drug. This time, the drug will be administered to volunteers suffering from the pathology for which the molecule was developed. This phase lasts a few years, during which the positive and negative effects of the therapeutic use of the active principle are analyzed – and its non-toxicity even in the patient’s disease[48]. Even at this stage, a large number of drugs fail the test and are abandoned.
Phase 3 – You bring the possible new therapy to the point. The drug is used with other drugs already developed for the same pathologies in order to statistically study the relationship between risk and benefit. The number of affected patients is increasing, reaching several thousand people. During this phase, which can last up to five years, the possible occurrence, frequency and severity of unwanted side effects are checked. Authorities (such as the FDA in the United States or the EMA in the European Union) only approve its distribution and marketing if the drug is effective and very well tolerated[49]. But the exams are not yet over.
Phase 4 – This phase is known as “post-market surveillance” because it is carried out after the market has been placed. This can take a few years and is useful for taking a closer look at the occurrence of rare side effects that did not occur in previous phases of the study[50]. It therefore happens with a certain frequency that the supervisory authorities decide in phase 4, in the light of serious events, to withdraw the medicinal product from the market and to prohibit its administration.
MediciNova and the MN-166 (Ibudilast)
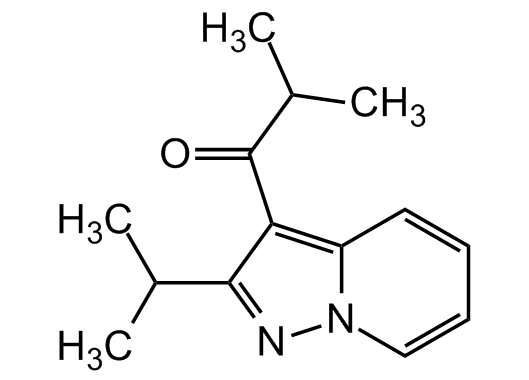
The ibudilast formula[51]
The great hope we are talking about is called Ibudilast (development code MN-166) and it is a drug offered by the Japanese company MediciNova for the treatment of amyotrophic lateral sclerosis (ALS) and progressive multiple sclerosis. It is a small molecule that inhibits the action of certain enzymes called phosphodiesterase (PDE) and is a macrophage migration inhibitor (MIF) factor[52]: in simple terms, ibudilast prevents the transmission of degenerative factors from a sick cell to a healthy cell, which will later be responsible for the death of patients. Chemical elements bind together according to certain rules that use phosphorus to move from one cell to another. If phosphorus is prevented from working (this is the hope that scientists hope), the disease will stop spreading[53].
These are things that we have known for some time. The product’s discovery as a phosphodiesterase inhibitor dates back to 1972 and is the work of two American doctors, Petko Uzunov and Benjamin Weiss[54]. In 1989, the Ibudilast patent was granted to Kyorin Pharmaceutical Company Ltd. Tokyo[55] has purchased and has already received the license to treat asthma[56]. Since among the side effects of the drug there is a property of relaxing blood vessels and dilating bronchial tubes, the drug has been used after asthma for dizziness after stroke[57]. The idea to analyze the use of ibudilast for multiple sclerosis came to Japanese doctors from a small lab called MediciNova Inc. in October 2004, to which Kyorin sold an exclusive license to develop and market the molecule in the world (excluding Japan, China, South Korea and Taiwan)[58].
MediciNova, a team of Japanese scientists from La Jolla, California and Tokyo[59], was founded in September 2000 as a subsidiary of the pharmaceutical company Tanabe Seiyaku Company Ltd. Osaka (now Mitsubishi Tanabe Pharma Corporation[60]) was born and is now listed both in the United States and on the Japanese stock exchanges. The project sponsor is Dr. Yuichi Iwaki[61], who specializes in analyzing the possibilities of drugs which he believes have a development possibility hitherto underestimated by the pharmaceutical giants. As a result, he’s partnered with small, highly specialized teams that have thrived in Japan this century to find niche markets in the vast ocean of the US market[62].
In technical jargon, MediciNova (MN) specializes in re-profiling drugs or transferring an existing drug to a new therapy plan through new studies[63]. MN is working on several projects: MN-001 (Tipelukast) against alcohol-free steatohepatitis and pulmonary fibrosis[64]; MN-221 (Bedoradrin)[65], MN-029 (Denibulin)[66], especially MN-166 (Ibudilast) for neurological disorders such as progressive multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), degenerative cervical myelopathy ( DCM), drug addiction (including alcoholism) and recently its use in the treatment of symptoms of Covid-19 is under investigation[67].
The intuition seems to be immediately correct. As early as late 1990, animal experiments showed that ibudilast can counteract calcium build-up in the central nervous system and the abdominal aorta – it inhibits phosphodiesterase so healthy cells don’t get sick[68]. In 1991, it showed its effectiveness against headaches because it was also a vaso-relaxant[69]. In 1993, it was confirmed to be beneficial in ischemic stroke[70]. Two years later, the efficacy of ibudilast in the treatment of circulatory disorders of the lower limbs of diabetics was observed[71].
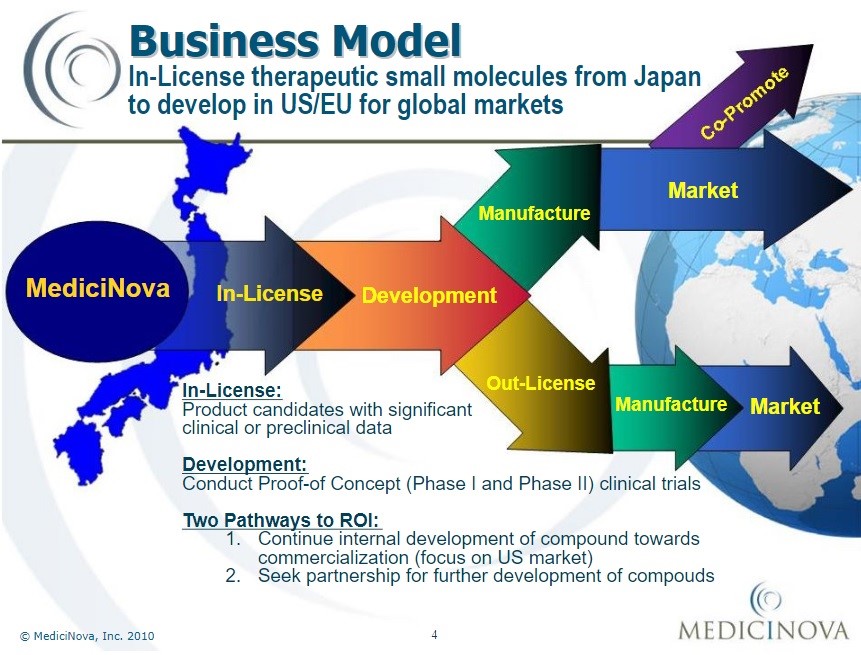
Business model of MediciNova Inc. since 2010[72]
The real breakthrough came in 1996 when a study found that the drug was able to counter the neurotoxicity of glutamate in cultured neurons in the rat hippocampus[73]. Glutamate, considered one of the most important neurotransmitters in our nervous system, has long been at the center of neurological diseases such as ALS. In some cases, the body overreacts to this enzyme, resulting in neurotoxicity and subsequent neuronal death. Ibudilast inhibits phosphodiesterase and therefore counteracts this dynamic[74].
In 1997, research confirmed its anti-inflammatory effects by weakening the infection of glial cells[75]. The results suggest that the researchers should test their therapeutic benefits in other respiratory diseases, and ultimately in neurological conditions such as ALS or the effects of drug addiction[76]. Soon after, further confirmations were received: in 1999, the drug was effective in the treatment of a rat disease, experimental autoimmune encephalomyelitis, making ibudilast a full candidate for clinical treatment. of patients with ALS[77].
The preclinical phase will take place in 2004 and the results are more than encouraging[78]. However, Kyorin wants to be able to market it as soon as possible and in 2007 focuses (with success) on the treatment of neuropathic pain: pharmacotherapy is generally based on the use of opioid analgesics, which are however only effective. modest and, on the other hand, have many side effects like addiction and abstinence syndromes. Ibudilast, which is used in combination with opioids, appears to have a dual effect: increasing the analgesic effect and significantly inhibiting the dependence syndrome[79].
This is when MediciNova enters the field, but with less than satisfactory results. A 2010 study of 297 patients with multiple sclerosis after 12 months was unsuccessful: “Ibudilast showed no beneficial effect on the rate of novelty of active lesions and relapses. However, early evidence suggests that ibudilast appears to be neuroprotective (…) and may have a positive clinical effect on the progression of disability”[80].
MediciNova does not give up the game and is experimenting with MN-166 (the name of Ibudilast in the catalog of Yuichi Iwaki’s laboratory), combined with other treatments: they don’t want to cure ALS, they just want to slow the progression symptoms. On this basis, the FDA (Food and Drug Administration) approved the start of the Phase 2 clinical study (IBU-ALS-1201) in 2012, the main objective of which is to assess the safety and tolerability of the drug. MN-166 in the EU for the treatment of amyotrophic lateral sclerosis[81].
In the same year, MediciNova and the University of Colorado signed a joint development agreement for the treatment of post-traumatic brain injury[82]. In 2013, MediciNova will be activated for a phase 2b (NCT01982942), with which the safety, tolerability and efficacy of ibudilast will also be tested in patients with progressive multiple sclerosis[83]. The study is being conducted in collaboration with the National Institutes of Health (NIH)[84], the National Institute of Neurological Disorders and Stroke (NINDS)[85], and the National Multiple Sclerosis Society[86]. Pharmaceutical America is starting to believe in the project.
Then come the positive results with the typical slowness of a procedure associated with such a terrible disease. In 2015, research found that alcoholic mice reduced their addiction by 50% when treated with ibudilast[87]. A team from another company, after careful preclinical analysis, found that although no decrease in inflammation was noted in multiple sclerosis, it is clear that ibudilast has a positive effect on the maintaining brain volume and therefore slowing the progression of the handicap[88]. In October 2016, the FDA granted MediciNova an additional seven years of exclusivity to conduct studies and experiments on this project[89].

An employee of MediciNova Laboratories at work
In March 2018, Iwaki decided to stop the drug treatment experiments – the results were too poor[90]. The FDA regrets the decision, saying that the results indicate that MN-166 “reduces cravings (and therefore addiction) and improves cognitive function“[91], and proposes to MediciNova to move to phase 2. In August 2018, MediciNova has announced the launch of a phase 2 study on MN-166 in degenerative cervical myelopathy in partnership with the University of Cambridge and the Cambridge University Hospitals NHS Foundation Trust[92]. The study focuses on the ability of the molecule to promote nerve growth in traumatic spinal cord injury. The study ends in triumph on March 19, 2020[93]. The results of the phase 2 study are very encouraging[94]: treatment with MN-166 slows the progression of the disease and also exerts a protective effect, which increases the risk of further inflammatory lesions is significantly reduced[95].
These results convince the FDA that the drug does have a future, and that is why phase 3 approval comes[96] despite the small lab lacking the legally mandated infrastructure to verify the results of each drug Administering any therapy , of any patient – this is called the “endpoint” in pharmaceuticals and determines the appropriateness of treatment[97]. However, the United States considers that sclerosis is so serious that in 2015, they decided to expand the network with products that are particularly promising for so-called orphan diseases, because they are still incurable[98]. To get phase 3, MediciNova must demonstrate that a significant number of patients are willing to take risks, and with sclerosis, finding them is not difficult[99]. The phase 3 clinical trial will begin in summer 2020[100].
In August, the American Psychological Association, which performed an additional test on MN-166, confirmed that ibudilast significantly reduced daily cravings in alcoholics[101]. Several months later, in December, the FDA approved the use of MN-166 in combination with another drug, beta interferon, to treat progressive multiple sclerosis[102]. In January 2021, MediciNova received a license from the Japanese authorities to use MN-166 in combination with riluzole (a medicine that prevents the negative effects of glutamate[103]) for the treatment of amyotrophic lateral sclerosis (ALS)[104].
A few days ago, MediciNova signed a contract with BARDA (Biomedical Advanced Research and Development Authority[105]) to test MN-166 as a countermeasure against chlorine gas-induced lung damage such as acute respiratory distress syndrome (ARDS) and acute lung injury (ALI)[106]. It looks like we are one step away from getting the license, one step away from being able to tell the thousands of new people with MS that there is real hope. But the race is still long, uphill and full of difficulties.
These endless hurdles
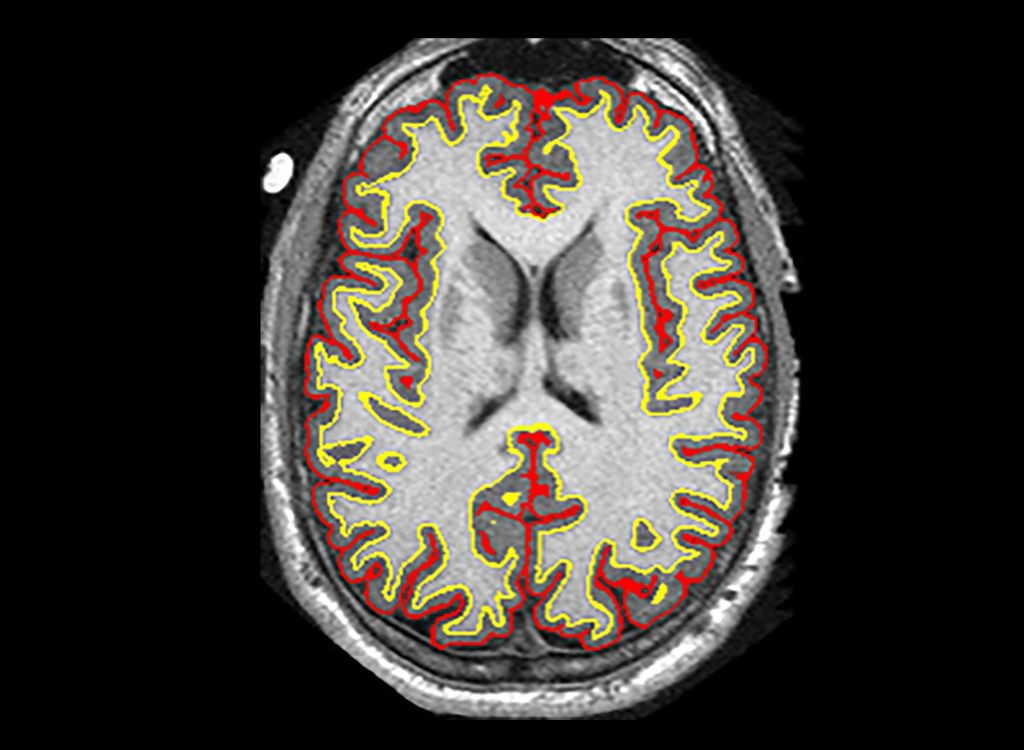
Phase II: Researchers found that a cluster of brain damage in patients was reduced by an average of 2.5 milliliters more than other treatments – a difference of about 48%[107]
The research and development of a drug entails enormous costs. According to a study published on the Jama Network in March 2020[108], it costs an average of $ 985 million to develop a new drug: from a low of $ 314 million to a high of $ 2.8 billion[109]. It can take up to 15 years for a pharmaceutical company to market its product. If you stop halfway because your money is gone, you will be left with nothing.
Competition is a critical risk. Often, several companies are working on a drug at the same time – and only one wins. So there is the risk of years of investing millions of dollars in the treatment and being overtaken in the last mile by a more effective drug developed by a better tolerated competitor, and even cheaper. The race is even tougher for small start-ups that don’t have a successful drug on the market: MediciNova recorded a net loss of $ 13.9 million in 2020 alone. cost since the start of the MN-166 study is already 382.9 million dollars. By the time MN-166 or one of the other newer drugs goes into production, things will get worse[110].
The truth is, MediciNova is drowning in debt. He reduced the number of employees from 25 (2009) to 8 (2019)[111], and the only hope that Dr. Iwaki has done everything to forge strategic alliances with large pharmaceutical companies who believe in the future of MN -166 and MN-001 – a misconception[112]. In our research, we contacted three multinational pharmaceutical industries. The answer was always the same: why should we come to an agreement and pay 100 for what we would probably only pay 10 after MediciNova went bankrupt? The result: the capital-hungry company decides to focus its strategy on the development of a vaccine against Covid-19 and announces in July 2020 the start of the development of a new drug with BioComo Inc. Tsu[113] and the institutional partner of Biocomo, the Mie University of Tsu[114].
But this drug does not work. In March 2021, MediciNova canceled the project[115] and replaced it with a partnership that started with BARDA (Biomedical Advanced Research and Development Authority), which pledged to provide financial and technological support for the development of MN- 166[116]. The announcement increases the value of MediciNova shares by doubling[117]. The money collected in the pocket is like manna from heaven. Meanwhile, MediciNova has launched the MN-221 for asthma and the version of MN-166 in combination with riluzole for the treatment of amyotrophic lateral sclerosis (ALS), as they are expected to receive a permanent license soon[118].
It’s a race against time because the patent expires in November 2035[119]. Since Ibudilast is a patent from 1989, it will almost certainly become a free patent like the one for Aspirin from 2039, and anyone can sell it, regardless of how much money MediciNova invests to develop it. In the United States, MN-166 is now administered in many teaching hospitals to patients who register to take responsibility for it. Multiple sclerosis is a disease that destroys the affected person in a slow, cruel and irreversible way. If you think of pharmaceuticals as the “Land of Toys” where anyone can make sky-high numbers without paying costs that list prices can justify, that’s a story to remember. And pray that ibudilast really works as it seems: with every lost day, human lives are lost.
[1] https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Worldwide%2C%20around%2050%20million%20people,60%E2%80%9370%25%20of%20cases.
[2] https://comitatoparkinson.it/i-numeri-del-parkinson-in-italia/#:~:text=%E2%80%9CMEDICINA%3A%20Parkinson%2C%20400%20mila,%C3%A8%20legata%20all’et%C3%A0%20avanzata.
[3] https://journals.sagepub.com/doi/full/10.1177/1352458520970841#:~:text=A%20total%20of%202.8%20million,gaps%20in%20prevalence%20estimates%20persist.
[4] https://www.karger.com/Article/Fulltext/493386
[5] https://www.sciencedirect.com/science/article/pii/B9780123750006000847
[6] https://www.sciencedirect.com/science/article/pii/B9780750614474500074
[7] https://www.sciencedirect.com/science/article/pii/B9780123750006000847
[8] https://www.sciencedirect.com/science/article/pii/B9780750614474500074
[9] https://microspedia.blogspot.com/2020/11/real-electron-microscope-nerve-cell.html
[10] https://michaeldmann.net/mann_i.html#:~:text=A%20neuron%20is%20a%20cell,of%20the%20cell%20to%20another.
[11] https://michaeldmann.net/mann_i.html#:~:text=A%20neuron%20is%20a%20cell,of%20the%20cell%20to%20another.
[12] https://michaeldmann.net/mann_i.html#:~:text=A%20neuron%20is%20a%20cell,of%20the%20cell%20to%20another.
[13] Gilles Clément, Angie Buckey, William Paloski, “Artificial Gravity – History of artificial gravity”, Springer Science & Business, Berlin 2007, pages 62-65; https://www.ncbi.nlm.nih.gov/books/NBK21535/
[14] https://www.newworldencyclopedia.org/entry/Soma_(biology)
[15] https://www.newworldencyclopedia.org/entry/Dendrite
[16] https://itechmedicaldivision.com/elettrostimolazione-e-contrazione-volontaria/
[17] https://www.newworldencyclopedia.org/entry/Axon
[18] https://www.syfy.com/syfywire/go-beyond-the-infinite-with-2001-a-space-odysseys-discovery
[19] https://www.newworldencyclopedia.org/entry/Axon
[20] https://www.kenhub.com/en/library/anatomy/the-myelin-sheath-and-myelination
[21] https://www.kenhub.com/en/library/anatomy/the-myelin-sheath-and-myelination
[22] https://web.archive.org/web/20111107033302/http://www.storiain.net/arret/num50/artic6.htm ; www.monci.it/homogubernator/Elettromagnetismo/2E_Pile_Zamboni.htm
[23] https://www.kenhub.com/en/library/anatomy/the-myelin-sheath-and-myelination
[24] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860500/
[25] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860500/
[26] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4191298/
[27] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4191298/
[28] https://alienanthology.fandom.com/wiki/The_Beast_Within:_The_Making_of_Alien
[29] https://www.rchsd.org/health-articles/lou-gehrigs-disease-als/
[30] https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Motor-Neuron-Diseases-Fact-Sheet
[31] https://www.policlinicocampusbiomedico.it/malattie/sclerosi-laterale-primaria#:~:text=La%20sclerosi%20laterale%20primaria%20%C3%A8,andamento%20dei%20sintomi%20%C3%A8%20progressivo.
[32] https://pubmed.ncbi.nlm.nih.gov/26515620/
[33] https://studiodiagnosticopantheon.it/latrofia-muscolare-progressiva-la-malattia-di-stephen-hawking/
[34] https://www.ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/amyotrophic-lateral-sclerosis-als-fact-sheet
[35] https://www.rxlist.com/fasciculation/definition.htm
[36] https://medlineplus.gov/ency/article/003188.htm
[37] https://www.aisla.it/vivere-con-la-sla/ipotesi-sulle-cause-della-sla/
[38] https://link.springer.com/article/10.1007/s00401-009-0545-9
[39] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4241182/
[40] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5536153/
[41] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2842587/
[42] https://pubmed.ncbi.nlm.nih.gov/21706151/
[43] https://pubmed.ncbi.nlm.nih.gov/17204932/
[44] https://toolbox.eupati.eu/resources/realizzare-un-farmaco-fase-6-fase-i-prova-del-meccanismo/?lang=it
[45] https://www.aifa.gov.it/sperimentazione-clinica-dei-farmaci
[46] https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
[47] https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
[48] https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
[49] https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
[50] https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research
[51] http://www.arisla.org/?p=8686
[52] https://www.openaccessjournals.com/articles/ibudilast-for-the-treatment-of-drug-addiction-and-other-neurological-conditions.pdf
[53] https://www.openaccessjournals.com/articles/ibudilast-for-the-treatment-of-drug-addiction-and-other-neurological-conditions.pdf
[54] https://chemport.cas.org/cgi-bin/sdcgi?APP=ftslink&action=reflink&origin=npg&version=1.0&coi=1%3ACAS%3A528%3ADyaE38XlsVyqtLw%3D&md5=93f10eb1f2cc78a01c54153040f0fdf4 ; https://www.sciencedirect.com/science/article/abs/pii/0005274472900605?via%3Dihub
[55] https://www.kyorin-pharm.co.jp/en/
[56] https://pubmed.ncbi.nlm.nih.gov/2556092/
[57] https://pubchem.ncbi.nlm.nih.gov/compound/3671
[58] https://materials.proxyvote.com/default.aspx?docHostID=322881
[60] https://www.mt-pharma.co.jp/e/
[61] https://www.crunchbase.com/person/yuichi-iwaki
[62] https://plus.credit-suisse.com/rpc4/ravDocView?docid=V6A35B2AK-e
[63] https://www.nature.com/articles/nrd.2018.168 ; https://plus.credit-suisse.com/rpc4/ravDocView?docid=V6A35B2AK-e
[64] https://medicinova.com/clinical-development/core/mn-001-nash/
[65] https://medicinova.com/clinical-development/core/mn-221/
[66] https://medicinova.com/clinical-development/non-core/mn-029/medicinova-approach/ ; https://www.sec.gov/Archives/edgar/data/1226616/000156459019002835/mnov-10k_20181231.htm
[67] https://medicinova.com/clinical-development/core/mn-166/ ; https://www.scienceboard.net/index.aspx?sec=sup&sub=Drug&pag=dis&ItemID=1749
[68] https://pubmed.ncbi.nlm.nih.gov/2257963/
[69] https://headachejournal.onlinelibrary.wiley.com/doi/abs/10.1111/j.1526-4610.1991.hed3107483_1.x?sid=nlm%3Apubmed
[70] https://www.tandfonline.com/doi/abs/10.1080/01616412.1993.11740130
[71] https://journals.sagepub.com/doi/10.1177/000331979504600808
[72] Slide Presentation (sec.gov)
[73] https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1440-1681.1996.tb02772.x
[74] https://lamenteemeravigliosa.it/il-glutammato-neurotrasmettitore-multifunzione/
[75] https://pubmed.ncbi.nlm.nih.gov/9396033/
[76] http://rsds.org/wp-content/uploads/2015/02/Rolan_Ibudilast_Review.pdf
[77] https://www.jni-journal.com/article/S0165-5728(98)00251-3/fulltext
[78] https://journals.sagepub.com/doi/10.1191/1352458504ms1070oa ; https://www.sciencedirect.com/science/article/abs/pii/S0028390803003721?via%3Dihub
[79] https://www.tandfonline.com/doi/abs/10.1517/13543784.16.7.935?journalCode=ieid20 ; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2662518/
[80] https://n.neurology.org/content/74/13/1033
[81] https://clinicaltrials.gov/ct2/show/record/NCT02238626
[82] https://connections.cu.edu/stories/cu-boulder-medicinova-collaborate-brain-injury-therapy
[83] https://clinicaltrials.gov/ct2/show/study/NCT01982942
[84] https://www.ninds.nih.gov/
[85] https://www.ninds.nih.gov/
[86] https://www.nationalmssociety.org/
[87] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017009/
[88] https://www.tandfonline.com/doi/abs/10.1080/13543784.2016.1221924?journalCode=ieid20
[89] https://alsnewstoday.com/news-posts/2016/10/18/ibudilast-mn-166-named-orphan-drug-for-amyotrophic-lateral-sclerosis-by-fda/
[90] https://www.globenewswire.com/news-release/2018/03/29/1455342/0/en/MediciNova-Announces-Results-of-Phase-2-Clinical-Trial-of-MN-166-ibudilast-in-Methamphetamine-Dependence.html
[91] https://www.researchgate.net/publication/297599603_Ibudilast_attenuates_subjective_effects_of_methamphetamine_in_a_placebo-controlled_inpatient_study
[92] https://www.globenewswire.com/news-release/2018/08/06/1547763/0/en/MediciNova-Announces-Initiation-of-NIHR-Grant-Funded-Phase-2-3-Trial-of-MN-166-ibudilast-for-the-Treatment-of-Degenerative-Cervical-Myelopathy-in-Collaboration-with-the-University-.html
[93] https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-004856-41/GB#A
[94] https://www.nejm.org/doi/full/10.1056/NEJMoa1803583?query=featured_home
[95] https://www.globenewswire.com/news-release/2008/09/18/385032/150688/en/Data-From-MediciNova-s-Two-Year-Phase-II-Clinical-Trial-of-MN-166-in-Multiple-Sclerosis-Presented-At-the-World-Congress-for-Treatment-and-Research-in-MS-WCTRIMS.html ; https://www.nejm.org/doi/full/10.1056/NEJMoa1803583?query=featured_home
[96] http://www.globenewswire.com/news-release/2018/09/25/1576125/0/en/MediciNova-Announces-Positive-FDA-Feedback-Regarding-Phase-3-Plan-for-MN-166-ibudilast-in-ALS.html
[97] https://www.sec.gov/Archives/edgar/data/1226616/000156459019002835/mnov-10k_20181231.htm
[98] https://www.fdanews.com/articles/172717-fda-indicates-flexibility-in-drug-development-for-rare-diseases?utm_source=Real%20Magnet&utm_medium=Email&utm_campaign=80249611
[99] https://www.regulations.gov/document/FDA-2015-D-2818-0002 “Rare Diseases: Common Issues in Drug Development Guidance for Industry” U.S. Department of Health and Human Services – Food and Drug Administration – Center for Drug Evaluation and Research (CDER) – Center for Biologics Evaluation and Research (CBER) – August 2015
[100] http://www.arisla.org/?p=8686
[101] https://www.biospace.com/article/releases/medicinova-announces-the-presentation-of-positive-results-from-phase-2-trial-of-mn-166-ibudilast-in-alcohol-use-disorder-at-the-american-psychological-association-2020-annual-convention/
[102] https://www.globenewswire.com/news-release/2020/12/29/2151193/0/en/MediciNova-Receives-Notice-of-Allowance-for-New-Patent-Covering-MN-166-ibudilast-for-the-Treatment-of-Progressive-MS.html
[103] https://medisoc.it/scheda-riluzolo/
[104] https://seekingalpha.com/pr/18155450-medicinova-receives-notice-of-allowance-for-new-patent-covering-combination-of-mnminus-166
[105] https://www.phe.gov/about/barda/Pages/default.aspx
[106] https://www.globenewswire.com/news-release/2021/03/09/2189908/0/en/MediciNova-Announces-Partnership-with-BARDA-to-Develop-MN-166-ibudilast-as-a-Medical-Countermeasure-Against-Chlorine-Gas-induced-Lung-Injury.html
[107] https://news.ohsu.edu/2018/09/05/study-suggests-potential-of-new-therapy-for-progressive-multiple-sclerosis
[108] Autore Olivier J. Wouters, PhD, Department of Health Policy, London School of Economics and Political Science, Houghton Street, London WC2A 2AE, United Kingdom.
[109] https://jamanetwork.com/journals/jama/article-abstract/2762311
[110] https://sec.report/Document/0001564590-21-006771/#ITEM_7_MANAGEMENTS_DISCUSSION_ANALYSIS_1 pag. 55
[111] https://www.macrotrends.net/stocks/charts/MNOV/medicinova/number-of-employees
[112] https://sec.report/Document/0001564590-21-006771/#ITEM_7_MANAGEMENTS_DISCUSSION_ANALYSIS_1 pag. 55
[113] http://biocomo.jp/english.html
[114] https://www.globenewswire.com/news-release/2020/07/27/2067757/0/en/MediciNova-Announces-SARS-CoV-2-Vaccine-Joint-Development-with-BioComo-and-Mie-University-Japan.html
[115] https://www.theglobeandmail.com/investing/markets/stocks/MNOV-Q/pressreleases/1242556/
[116] https://drive.hhs.gov/ReDIRECT.html
[117] https://www.nasdaq.com/articles/medicinova-partners-with-barda-to-develop-chlorine-gas-induced-lung-injury-treatment-stock
[118] https://seekingalpha.com/pr/18155450-medicinova-receives-notice-of-allowance-for-new-patent-covering-combination-of-mnminus-166
[119] https://alsnewstoday.com/news-posts/2019/01/23/medicinova-closer-to-us-patent-ibudilast-rilutek-combo-for-als-neurodegenerative-diseases/
Leave a Reply